3 tips to manage medical device risk and safety
The development and marketing of medical devices is highly regulated and many pitfalls exist, potentially complicating and increasing costs. Here are 3 key questions to begin.
As a test house, FORCE Technology is seeing a growing demand for accredited approvals. The increasing demand clearly demonstrates that requirements are tightening, and the control functions are working as intended.
For manufacturers of medical devices it is important to notice that the regulation is not simply a 'bad thing'. The regulation helps keep the market free of unacceptable - at times dangerous - products by setting the bar high for approved marketing. At the same time, the relevant standards contain excellent process-oriented methods that ensure the efficient execution of development projects with a few reversals.
The best approach to regulation and standards is to strive to achieve compliance from the outset of any development project. All too often, companies experience a relatively heavy burden of backlog in the form of lacking documentation, incorrect labelling, lack of security mechanisms, etc., and the result is increased costs and delays as a result of necessary updates and the need to repeat costly accredited tests.
3 central questions
Initially, any manufacturer of medical equipment should ask a number of clarifying questions:
- What is the intended use of the product? (Curing of a disease? Remedial treatment, relief or monitoring, examination of a specific disability? Contraception? On which body part is the product to be used?)
- Who should use the product? (Gender, age, demographics, disability and any restrictions. Consider these for all user groups - installers, healthcare assistants, relatives, patients, etc.)
- Where is the product to be used? (at home, in hospital, in home care, during transportation, in emergency vehicles, on aircraft, helicopters, etc.)
These are very important questions. Let's go through them one at a time.
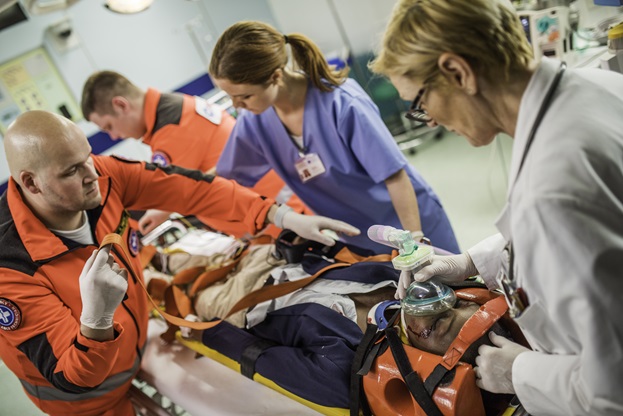
1. What is the product's intended use?
The intended use forms the basis for classification as a medical product. It is imperative that the classification is in place early on, as an elevated medical classification may place additional requirements for the underlying development processes, product requirements and documentation. The classification is made on the basis of the “intended use” and the communication with which the product is promoted.
It is essential that the intended use and communication are consistent. As an example, the difference between the statements “The chair supports the lower back” and “The chair supports people with problems in the lower back” will determine whether or not one is dealing with a medical product.
When describing the intended use as a manufacturer, one should clarify how one specifically aims to prove that the product actually works according to its intended use. Therefore, it should be ensured, as far as possible, that the intended use is formulated in a measurable form, since the effect of the product must be shown in the form of a clinical evaluation - e.g. in user trials before marketing.
The clinical evaluation includes literature studies on the effect of similar products as well as analysis and assessment of which safety factors have played a role in other similar products. It may be unlawful to market non-state-of-the-art medical products whose deficiencies when compared to competing solutions pose an increased risk to the user.
On the flip side, there is obviously very little advantage in studying competing solutions or the safety-related conditions of competing solutions, as one’s own design is largely complete, and incentives for improvement are therefore typically very limited. Some companies choose to enter the market with the anticipation of an update in the near future. They often don’t realise that an update may very well require renewed approvals and updating of large amounts of documentation. It is highly recommended to adhere to the standards for User-friendliness (IEC 60601-1-6 / 62366-1) and EN ISO 14155-1 regarding clinical study, partly to ensure compliance with the standards, but more importantly to plan and streamline the development process and ensure that all relevant data is collected as early as possible for the safety and quality of the product.
2. Who are the users of the product?
The users of a piece of medical equipment are often not only patients, but also include pharmacists or hardware dealers, freight carriers, installers, hospital porters, nurses, relatives or others who come into contact with the product during the product’s life cycle.
The role of the user in relation to product safety must be considered. A hardware dealer with inadequate knowledge of the product may sell a variant that is too powerful, an installer may set up the equipment incorrectly, a porter may not be sufficiently disinfecting the product, a nurse may set up the product incorrectly, and relatives may inadvertently activate a function, etc.
Identify user situations and risks
It may be a good idea to make a number of sketches or descriptions that illustrate the product in various situations where users and their interaction with the product are identified. Such a “user history” can create a basis for assessing risks, needs for education, training and labelling, as well as content for user manuals or technical documentation. The user history thus helps to define the safety requirements for the product. A good user history can also provide a good framework for uncovering functional requirements that cover the entire product life cycle.
Uncover user restrictions
Thorough uncovering of user restrictions can help reduce risks and ensure that the product can perform its intended use across the entire user group. For example, assessments can be made as to whether the user has sufficient:
- motor function to operate the product,
- cognitive understanding to understand the operation of the product,
- faculty of vision to discern the features of the product,
- education to read and understand the user manual.
If a user cannot be presumed to have sufficient capabilities, such as in the case of medical products for home use, further securing of the product, illustrative labelling or contraindications to the healthcare assistant or seller may be required in the form of warnings regarding restrictions. In all cases, such requirements must be managed in relation to risk management, and if there are risks, the impact of the remedial measures must be verified through user studies.
3. Where will the product be used?
Humidity, high or low temperature, high or low pressure, rough handling and electromagnetic interference are all examples of mechanisms that impact a product resulting from the surrounding environment of the product. The environment - unlike users - cannot be managed or controlled, and the product must therefore be designed robustly in order to carry out its intended use correctly under the influence of the surrounding environment throughout its lifetime.

Since the robustness of the design depends on design elements, which are designed to protect the product from environmental influences, it is important that potential risks are uncovered before the design is established. To the extent that the impact can lead to safety risks for users, risks must be identified and documented in the product’s risk analysis. The uncovering includes a flow that entails to:
- identify the product characteristics that may be affected by the environment and pose a safety risk,
- describe and treat cause and effect,
- evaluate probability and severity of harm (risk level),
- planning of risk mitigation measures,
- implement and verify the effect of the measures,
- assess the remaining risk level and any new risks arising from the measure.
The standards not only serve as inspiration but set specific requirements that are best met if you initially plan and design with the requirements in mind. Your test house will ask you for documentation of the uncovering of those risks that are covered by the standard to which the test house is testing for have been performed. Creating the documentation is a heavy, time-consuming and elaborate process that provides little value and extra hassle if created at the tail end.
(This article was published in Medicoteknik, September 2019)




