Approval of medical devices with radio
Risk management is essential for the approval of medtech devices.
The requirement for EMC risk considerations for medical devices is evident in the harmonised standards. But how do these risk considerations work in conjunction with the performance requirements for radio equipment, and how are tests best optimised to ensure compliance with the Medical Device Directive and the Radio Equipment Directive when dealing with medical devices with Bluetooth, Wi-Fi or other built-in radios?
Manufacturers of medical devices with built-in radios must ensure and declare that their equipment meets the requirements of both the Radio Equipment Directive (RED) and the Medical Device Directive (MDD) DIR93/42/EEC. In May 2020, the latter will be replaced by the Medical Device Regulation MDR2017/745. But the combination of requirements from two worlds that handle compliance fundamentally differently provides high complexity in test planning.
The addition of a radio in a medical device has implications for both for the risk management and for what to look for during testing – the appliance’s performance criteria. Careful planning of the testing of a medical product with radio is required, so that all relevant functions are monitored simultaneously and adequately, not to have first to test the compliance with MDD and afterwards test again to ensure compliance with RED.
Testing for EMC is not straightforward
When testing for electromagnetic compatibility, it is not possible to test for the combined requirements from MDD and RED. If the equipment has a built-in radio, it is necessary to reduce the pass criteria for the medical part. And this is always the case, as the adding of radio gives rise to the definition of several performance criteria and considerations of these in the risk management file of the equipment (typically prepared according to ISO 14971 for medical devices).In the EN 60601-1-2 standard, that deals with electromagnetic compatibility for medical devices, the concepts of Essential Performance and Basic Safety are used. The Basic Safety criterion means that no physical danger may arise as a result of a single fault during normal operation of the equipment. The Essential Performance criterion is about maintaining that part of the equipment's clinical functionality, where it is associated with an unacceptable level of risk, should this functionality fail.
The environment determines the EMC test levels
The levels at which EMC are to be tested are specified in the current version of EN 60601-1-2 (ed. 4) based on the environment in which the equipment will operate. This can be a home environment, clinical or special environments such as ambulances or in aircraft.Failure to comply with the standards definitions of test levels in a predefined home or clinical environment means that by definition, you are in a special environment. In special environments, risk analysis is required for both test levels, the tests to be conducted, and the extent of these tests. Thus, a significant increase in the number of issues to be addressed in the electromagnetic compatibility section of the risk analysis.
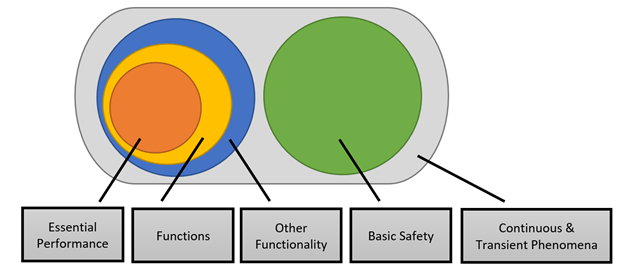
The performance criteria under RED
When assessing radio appliances (which, after all, must comply with RED), the performance criteria are viewed quite differently. As regards the EMC requirement, RED is concerned with ensuring durable radio communication, taking into account the operation of other appliances. Not as MDD, which is concerned with hazards.In the ETSI standards, the manufacturer is asked to define the parts of the radio appliance’s function that must function without being affected by Continuous Phenomena and to define the parts of the function of the equipment that must function without being affected by Transient Phenomena in regard to transmitting and receiving operations as well as the other performance of the equipment. This is illustrated in Figure 1.
When to do EMC test?
One can rationalize that a medical device does not always have Essential Performance. This applies if all clinical function is acceptable to fail if the equipment fails – for example, during EMC testing.For equipment that is so simple that even Basic Safety - according to the manufacturer's risk analysis - cannot be compromised during EMC testing, there is really no reason for EMC testing for immunity, and the manufacturer can, in principle, try to convince its Notified Body of the adequacy of this strategy. However, when introducing a radio into the equipment, according to RED, it is required to test and monitor 'everything' defined to a sufficient level of operation.
Complexity arises in particular when the radio function of a medical device is considered to be associated with an unacceptable risk to the clinical functionality if the radio function fails or degrades. But no matter what, individual product EMC-related risk analysis will always be required to determine the performance criteria to comply with both MDD and RED.


