Sustainable medical devices: from design through refurbishment and to recycling
Medical products, particularly single-use equipment, demand large quantities of resources and come with a major carbon footprint. This begs the question: How can we develop and purchase healthcare products that work optimally, are safe, and do not use any unnecessary resources?
IEC 60601-1-9, titled "Medical electrical equipment - Part 1-9: General requirements for basic safety and essential performance - Collateral Standard: Requirements for environmentally conscious design," suggests actions to take at various stages of a product's life cycle to reduce its environmental impact and make it more sustainable.
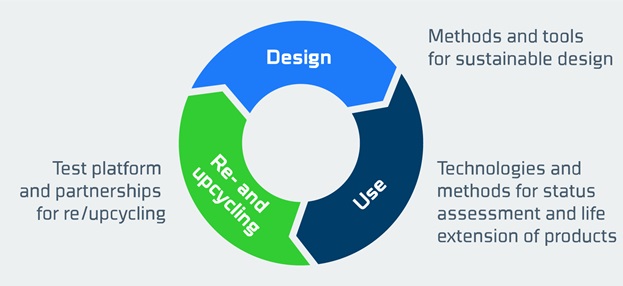
The most effective approach is to consider sustainability requirements early on in the design phase or when soliciting tenders. Experience has revealed three important areas to focus on in this context:
- Long lifetimes
- The choice of materials
- Built-in lifetime extension options
Products with long lifetimes in their original form make the most of the resources i.e. materials and energy used to produce them. Lifetimes, expressed in years or number of uses, should be considered relative to the period for which it makes sense to use a product. The choice of components, a form factor suitable to the expected usage environment, and general design practices are important factors to consider when designing long-lifetime products. To ensure that a product's developers have succeeded in creating a safe product that functions optimally, its lifetime can be verified through analysis and testing. For buyers of medical products, it is important to ensure that lifetime is listed in tender materials and documentation.
From single-use to multiple-use products
Naturally, single-use products have limited lifetimes - but if we are serious about sustainability, we ought to also consider whether these products can be replaced with multiple-use products by changing materials and facilitating repairs or component replacements, allowing at least portions of these products to be reused. Choosing the right materials e.g. replacing plastic with metal can potentially extend the lifetime of a device from five or six uses to five or six years. Of course, risk assessments are always needed.
Choosing the right materials and combining them appropriately is key to product sustainability, not only in terms of the manufacturing process's environmental impact, but also in terms of waste sorting and recycling at the end of a product's lifetime. Packaging material is also important to consider in the context of sustainability.
Last, but not least, it is important in the design phase to prepare possibilities for extending a product's lifetime, or to specify and assess such possibilities in the procurement process. Examples of such possibilities include the ability to replace parts subject to wear and parts with limited lifetimes, such as motors, fans, pumps, and power sources.
Focus on developing methods and tools for sustainable design
In FORCE Technology's performance contract project entitled "Long Live Products and Materials," we focus on developing methods and tools for sustainable design, including long lifetime and possibilities of lifetime extension, technologies and methods for status assessment/condition monitoring, and re- or upcycling of medical devices.