Ensuring market access for sustainable and safe medical devices
MedTech and welfare tech firms tackle challenges adopting new technological and sustainable solutions, ensuring compliance with top-priority standards, regulations, and consumer safety.
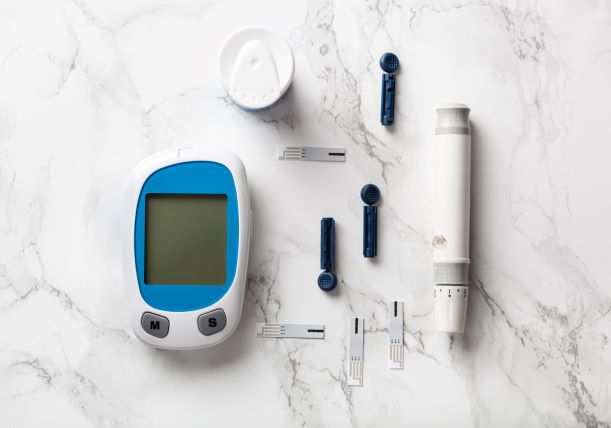
Time-to-market is a challenge for MedTech and welfare tech products
Time-to-market is crucial for the competitiveness of companies producing devices, products, or equipment within MedTech and welfare. Short R&D cycles, rapidly evolving technology, growing risk awareness and increased user involvement all contribute to the need for more innovative use of technology to ensure fast launches of products. And when it comes to connected devices, e.g., remote monitoring or app-based solutions, cyber security is key.
But, in a heavily regulated industry such as MedTech and welfare tech, it can be challenging to implement new technological and sustainable solutions when compliance with standards, regulations and consumer safety remain the highest priorities.
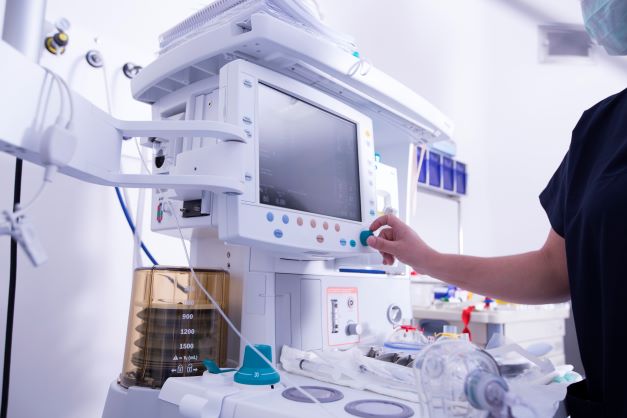
An easy and safe way to customers and end users
That is why we strive to make innovation, development, test and launch of new medical devices and equipment easy and safe. To do this, we apply our impartiality and technological expertise, combined with regulatory knowledge and active participation in standardisation committees, and we ensure access to a wide range of testing facilities.
Shorter time-to-market without compromising quality
We ensure market access through:

Product approval of medical devices
Ensure your medical device’s compliance with regulatory requirements, such as MDR, already in the RnD phase.
Chemical test of equipment, incl. PPE
Testing and approval for importers, manufacturers and distributors that documents your product’s compliance with existing market standards.
Life cycle assessment (LCA) of products
Life Cycle Assessment makes it possible for you to document and explain how your product or service impacts the environment.
Test and certify any hearing solution for any market
Ensure compliance, testing, and global access for your hearing solutions.
Material testing validates Exo360's fracture care
/Case

Medical device designed to preserve donor hearts
/Case
How to prepare the regulatory approval of a medical device to preserve and transport donor hearts.

From single test to dedicated partnership with WSA
/Case
Hearing aid giant gets documentation for compliance with new regulations and market requirements.

Knowledge of standards future-proofs medical devices
/Case
XO CARE uses knowledge about standards actively to future-proof their dental products.
Learn more about safe and sustainable medical devices

Green design of medical devices
/Article
How to use the standards to develop green design of your equipment.

IoT as driver for a digital health sector
/Article
IoT will affect our health sector. This requires focus on responsible design and reliability.
Risk and safety management for medical devices
/Article
How to handle risk and safety management when you develop medico devices.

What are the life science industry’s biggest technological challenges?
New report maps the technological challenges with digitalization and green transformation in life science companies.
For more information, contact
Kasper Brix Adsersen
Senior business development manager
T: +45 42 62 79 52