Document that your hearing solution is compliant with standards and market requirements
Get help to test, certify and document any hearing solution to ensure market access.

70% of hearing solution approval processes need to start over due to inadequate documentation
The regulatory process for hearing solutions like traditional and OTC hearing aids, earbuds and lifestyle wearables is complex and full of pitfalls. Small to medium-sized manufacturers often face significant challenges, leading to incomplete documentation, inadequate testing and unmet market-specific requirements. Consequently, 70% of products must restart the approval process, incurring additional time and financial costs and delaying product launches. Compliance with new FDA standards adds further difficulty for OTC products, requiring a meticulous and hands-on testing approach.
We approve about 80% of all hearing aid devices on the market
We specialise in testing and documenting the standard compliance of hearing aid devices, covering technical audiology, acoustics, electromagnetic compatibility (EMC), reliability and electrical safety. Annually, we issue over 700 detailed reports confirming these devices' quality and durability for global manufacturers. Our comprehensive evaluation process is pivotal in approving approximately 80% of all hearing aid devices launched on the market. Beyond test results, we provide invaluable guidance tailored to your product's regulatory journey, leveraging our expertise to expedite market entry while maintaining uncompromising quality.We can help you faster, safer and cheaper through your regulatory process
We can contribute to shaping your market access strategy by offering pre-testing during the design phase and mapping out the scope of requirements and documentation levels. We guide you through the documentation and test process every step of the way.
Get access to a full package of testing to relevant hearing product standards
Based in Denmark, home to some of the world's leading hearing aid companies, we have contributed to developing standards for hearing solutions for decades. We are proud to be among a select group of test houses with the facilities, expertise and accreditations to offer a broad spectrum of services tailored to support hearing product manufacturers in gaining global market access.
Which hearing product standards do we test and certify according to?
Contact us today to save time and money with specialised hearing solution testing and documentation
For more information, contact
Compliance & Product Testing
Customer Centre: Consultancy, test and product approval
T: +45 43 25 14 50Testing capabilities for hearing aids and hearing solutions

Reliability tests
Tensile, drop, mechanical shock and vibration, thermal shock (high/low temperatures and humidity), pressure, biocompatibility standards (ISO 10993), toxicological harmful chemicals, harmful substances such as phthalates and latex, wear testing, artificial earwax testing, sweat testing, battery compartment testing, dust testing (IP IEC 60529), and battery life testing.

Sound quality tests
Using SenseLab, a world-leading, widely recognised evaluation system with a trained listening panel and facilities meeting the highest standards.
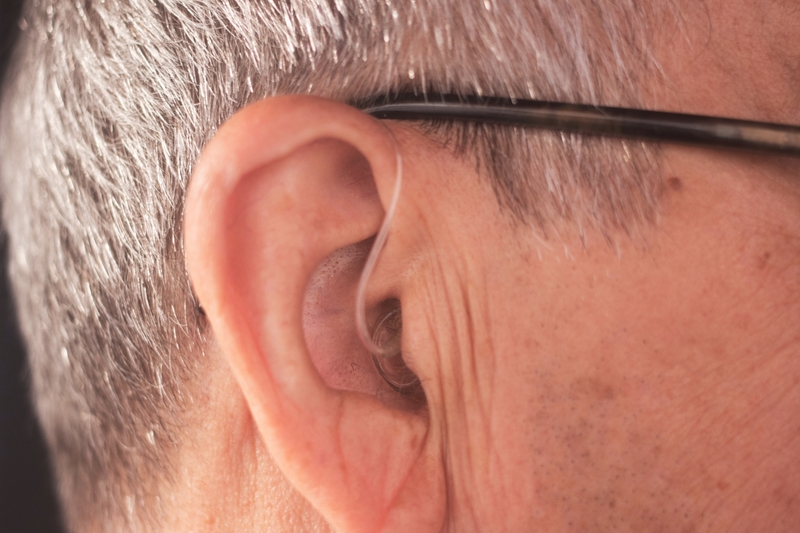
Hearing aid battery testing
Primary and secondary batteries: IEC 60086-1, -2; EHIMA recommendations for Zinc-Air Hearing Aid Batteries. Adjacent management systems and quality management systems for medical device manufacturers, including requirements for documenta-tion, traceability, risk management, process and product validation, helping to comply with industry and regulatory requirements.

Certification of services and compliance documentation
Medical Device Regulatory (MDR); FDA; EC; IECEE CB Scheme via FORCE Certification. To guide you through the extensive regulatory framework for market access, we have a specialised Approval Management Team ready to assist with coverage, definition, composition of approval documentation and project management to ensure that the enabling markings and declarations of conformity, as well as all other parts of the documentation basis, are in place.

Case
From single test to dedicated partnership with WSA
Market access for safe medical devices
/Page
We strive to make development, test and launch of new medical devices and equipment easy and safe.
Expert medical device testing services
/Service
Comprehensive testing and certification of medical devices ensure compliance and market access.

Technical audiology
/Service
Testing of hearing aids and audiological equipment, approval of clinics and research/training.
