Hygienic design rules that benefit pharma production
With good and relevant guidelines, the food industry has a ground set of rules for hygienic design that the pharma production may benefit from.
European Hygienic Engineering and Design Group (EHEDG) was established in 1989 and has since then provided hygienic design guidance for the food industry mainly in the form of balanced guidelines covering a wide range of hygienic design topics.
Since 2000 EHEDG the guidelines are supplemented by a certification scheme at first merely for components used in closed processes intended for cleaning-in-place.
The certification scheme has been extended to cover more areas along the way and the focus of the guidelines has moved from mainly a components perspective to being more holistic and focusing on production plants, process lines, cleaning procedures and sound process execution.
Even though EHEDG is initially intended to benefit the food industry specifically many principles may have validity for a broader audience e.g. when aseptic or sterile production is the focus. In principle hygienic design is intended to make processes easier to clean but also to limit the risk of buildup of biofilm which is equivalent to the risk of retaining allergens.
The processes of retaining biological and organic material may be different in their physical and biological/chemical nature but the process design requirements may very well be similar.
The foundation for proper hygiene
Product safety is non-negotiable when producing products for human intake, however hygienic design provides more benefits to more than the end-user.
The result is often improved product quality, reduced environmental impact and improved process-efficiency due to reduced maintenance costs and optimal cleaning processes. In many ways hygienic design specifications capture key essential aspects of product safety, which if not monitored and managed properly during planning, design, installation and commissioning may hamper the ability of a process plant / line to provide the intended high-end food products.
Applying sound hygienic principles in the construction of production lines, having an efficient cleaning procedure, a valid cleaning validation and proper qualification procedures to test that specifications are met are all important to achieve good hygienic performance for food as well as pharmaceutical companies.
The currently 44 EHEDG guidelines plus a few additional documents covers the following areas:
- General Principles, Materials, Surfaces
- Test Methods
- Factory Design incl. Design of Utility Systems
- Open Equipment
- Closed Equipment for Liquid Food
- Closed Equipment for Dry Particulate Materials
- Packaging Machinery incl. Filling Machinery
- Heat Treatment
- Cleaning & Validation
New guidelines are to a higher extent focusing on the holistic approaches in processes e.g. issues pertaining to Factory design, process line design, utility systems, process management and validation of the hygienic status of final installations
EHEDG Certification scheme
EHEDG certification follows two main classes wet processing called EL and dry processing called ED. Both classes apply a sub notation labeling equipment suitable for cleaning in place Class I and equipment which needs to be dismantled during cleaning Class II. These are the only two options available for ED (dry processing) whereas the EL scheme (wet processing) also include the certification Aseptic and AUX for auxiliary equipment. Thus, yielding four main groupings of certification:
- EL Class I and II
- EL Class I and II Aseptic
- EL Class I AUX
- ED Class I and II
The prerequisites for certification are compliance with the relevant EHEDG guidelines and the basic criteria set down in Guideline no. 8 (available for free download) these being:
- Food contact materials must comply to EU regulations and FDA
- Surfaces must have a roughness lower than Ra = 0.8 µm and must be free of imperfections
- Rounding radii of a minimum of 3 mm must be applied in corners (3-A specify 3,2 mm) or the angle must be open which requires an angle of 135⁰ or above.
- Surfaces must be drainable and a slope of minimum 3⁰ is required.
- Gaskets must be flush with the adjacent materials and
A certification process is carried out by one of 8 EHEDG Accredited Test Laboratories and here initiated by an Authorized Evaluation Officer (AEO) who initially will inspect drawings and a physical component and, in some cases, defer the component to testing.
Hygienic integration and project management
Using hygienically designed components (preferably EHEDG certified) is only part of the process.
Hygienic integration the process which combines two hygienic entities together whilst assuring that the assembly is hygienic and meets the requirements for further integration steps. In the final stage this will be the integrated process line.
The process of getting from a set of user specifications for a process line to a properly working process line includes many activities and if this process is not formalized there is a risk that hygienic performance may be challenged. Such a formalized model is The Lifecycle Development Model or V-model due to the characteristic ‘V’ shape presented in figure 1.
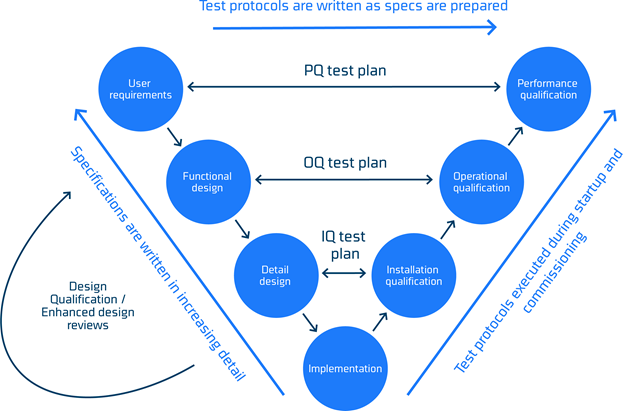
The model allows for refinement and documentation of specifications and a loop back to ensure that user requirements are revisited, and testing protocols updated accordingly.
The V-model is a framework comprising design (left hand side), execution (bottom) and commissioning (right hand side) of a project.
The model provides a logical sequence that organize the complex activities of defining a project scope, executing it and finally qualifying it.
The qualification is critical for the hygienic performance. It is important that the performance qualification testing plan are adequately designed to test against the intended requirements otherwise the possible challenges in hygienic performance may not be reviled.
FORCE Technology is EHEDG Accredited Test Laboratory