Ensuring hygienic integration and cleaning efficiency in the dairy industry
In the dairy industry, hygienic design and effective cleaning procedures are critical to ensuring product quality and safety.
Hygienic design, efficient cleaning procedures, and thorough validation are essential for achieving top hygienic performance in the dairy industry—from farms to processing facilities.
Hygienic design must limit the buildup of biofilm and those that are formed during the production time must be effectively removed by the cleaning procedure, otherwise leftovers of biofilm may contaminate the raw or pasteurized milk with thermoduric bacteria.
Biofilm in the dairy industry
The presence of biofilm in the dairy industry it is equal to a higher number of thermoduric bacteria.
The thermoduric bacteria are a heterogeneous group of bacteria that can survive the pasteurization and will carry over into the dairy products causing defects in the final products. Such as reduced shelf life for milk or spoilage of butter and cheese. Especially for the mature cheeses the problems can be severe.
Because of the maturation and “the long shelf life” of these cheeses, even a small change in the diversity of the microorganisms in the raw or pasteurized cheese milk will results in abnormalities during ripening. Causing change in the desired taste which will be more and more pronounced during ageing. Problems like gas production or colored spots can also be observed.
Optimised cleaning procedure
The cleaning procedure in the dairy industry is Cleaning in Place (CIP) for the closed equipment (pipes, tanks, pumps etc.) and manual cleaning for the open equipment. For both cleaning types Sinners circle is important (see figure 1).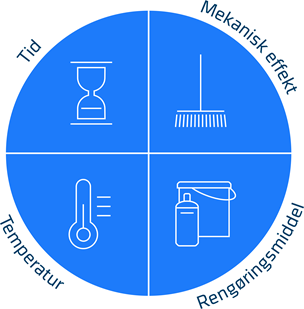
The 4 parameters are:
- Temperature which must be optimized to cleaning chemicals and organic materials on the surfaces.
- Time which must be long enough to remove all formed biofilm on the surfaces. Note that longer production times leads to formation of more biofilm and that higher count of thermoduric bacteria in the raw milk leads to quicker formation of biofilm in the dairy.
- Chemistry where it is important to choose proper cleaning chemicals depending of the organic material present.
- Mechanical effect which may be the most difficult parameter to get right in cleaning procedures (both manual cleaning and CIP). The mechanical impact in open equipment is the effect on the surface from brushing, scrubbing, or low-pressure foam cleaning. In closed equipment this is the effect of fluid flow on the surface of the integrated production line. The mechanical effect needs to reach all parts of the equipment i.e. angles, corners, cervices etc. Surfaces must be as smooth as possible to secure proper effect of the mechanical action. Welds and gaskets in joints must be flush with the surface. Poor welds or gaskets which protrudes into fluid flow will lead to buildup of organic material and will in time lead to poor cleaning.
Balancing these parameters ensures thorough cleaning, reducing biofilm buildup and improving product safety.
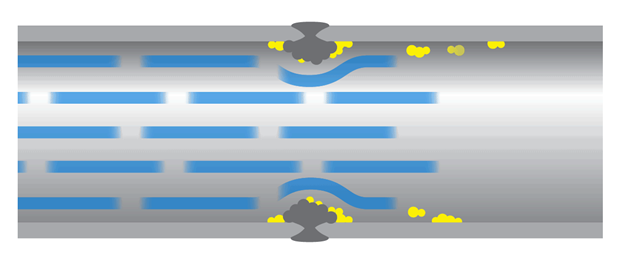
Hygienic integration
Hygienic integration involves combining components in a way that maintains cleanliness and meets strict hygiene standards throughout the production process. In the final stage this will be the integrated process line.
The process of getting from a set of user specifications for a process line to a properly working process line includes many activities and if this process is not formalized there is a risk that hygienic performance may be challenged.
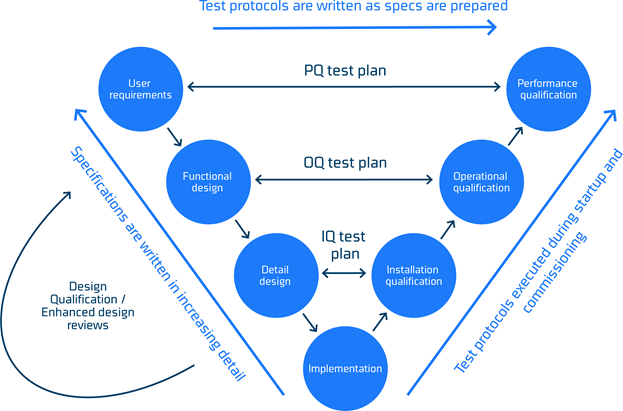
Such a formalized model is The Lifecycle Development Model or V-model due to the characteristic ‘V’ shape presented in figure 3. The model allows for refinement and documentation of specifications and a loop back to ensure that user requirements are revisited, and testing protocols updated accordingly. The V-model is a framework comprising design (left hand side), execution (bottom) and commissioning (right hand side) of a project.
The model provides a logical sequence that organize the complex activities of defining a project scope, executing it and finally qualifying it. The qualification is critical for the hygienic performance. It is important that the performance qualification testing plan are adequately designed to test against the intended requirements otherwise the possible challenges in hygienic performance may not be reviled.