Emissions from ship engines using methanol as a new, green fuel
Transitioning to new, green fuels for ship engines, such as methanol, may have a positive impact on the climate in the long term by significantly reducing CO2-emissions.
The International Maritime Organisation (IMO) has announced a series of targets for significantly reducing the emission of greenhouse gases from ship engines in the decades to come [1]. The target for 2030 is a 40% reduction in carbon dioxide (CO2) emissions per unit transport work, ultimately scaling to climate neutrality around the year 2050. Presently, fossil fuels such as marine diesel and heavy fuel oil are the dominant fuels used in ship engines, resulting in significant CO2 emissions. Existing battery technologies cannot currently support the electrification of international shipping.
Possible new, green fuels for ship engines
Candidates for potential climate-neutral fuels for shipping include hydrogen (H2), ammonia (NH3), and methanol (MeOH). Of these, MeOH appears most viable in the short term, given that a variety of existing ship engine types could be adapted to burn MeOH [2].
Up until now, the shipping industry has been a major emitter of a lengthy list of undesirable components. However, only nitrogen oxides (NOx), sulphur oxides (SOx), and CO2 are regulated by the IMO. In 2018, shipping emissions amounted to 20.2 Mt NOx, 11.4 Mt SOx, and 919 Mt CO2 [3]. Those CO2 emissions made up 2.89% of global anthropogenic CO2 emissions in 2018 [3]. Additionally, other components emitted by ship engines can also impact the climate, human health, and local environments, including various greenhouse gases, carbon monoxide (CO), soot particles, and other organic compounds emitted as gases or particulates [3].
The need for new, green fuels with no harmful emissions for ship engines
One important consideration is ensuring that the green shipping fuels of the future do not result in significant emissions with undesirable effects on human health, local environments, or the climate.
This article focuses on emissions associated with the combustion of MeOH in ship engines. Ideally, complete combustion of MeOH results in the emission of just CO2 and water vapour, via the following reaction:
2 CH3OH + 3 O2 -> 2 CO2 + 4 H2O
In practice, MeOH is difficult to ignite in a combustion engine without the use of a pilot fuel. As a result, both pilot fuels and lubricant components could contribute to the emissions generated when MeOH is burnt in a combustion engine.
Assuming that net carbon-neutral production, transportation, storage, and combustion of MeOH is possible, taking into account the use of pilot fuels and lubricants, then at least in principle, climate neutrality is possible [4], provided that the process does not otherwise involve emissions with a warming of the climate.
The effects of atmospheric methanol on the climate
After methane (CH4), MeOH is the most commonly occurring hydrocarbon in the atmosphere. Emissions of MeOH are associated with both natural and anthropogenic sources [5, 6].
While MeOH itself is not a greenhouse gas, MeOH can indirectly contribute to global warming. MeOH typically breaks down in the atmosphere when it reacts with the hydroxyl radical (OH). The primary process that breaks down atmospheric methane, a potent greenhouse gas, is also a reaction with OH [7]. Consequently, significant emissions of MeOH will result in climatic warming by reducing the concentration of OH available to break down methane.
Furthermore, the final decomposition products of atmospheric MeOH include CO2. As a result, there must be a major focus on avoiding emissions of unburned MeOH whenever possible to minimise the climatic impact of MeOH as a fuel.
Assessment of emission effects is complicated by atmospheric processes
The emissions from combustion engines can generally be divided into gases and particulates. However, there are many potential components of emissions that undergo processes in the atmosphere that can change their properties in terms of their effects on the climate, human health, and local environments.
Given this, chemical atmospheric processes can complicate the classification of possible effects of emissions in the atmosphere; see some examples below.
The climatic impact of ship engine emissions
The many potential constituents of combustion engine emissions can contribute to global warming either directly, as in the case of greenhouse gases (e.g., CO2, CH4, and N2O), or indirectly, as precursors of CO2 (e.g., CO, MeOH, and CH2O) and of ozone (e.g., hydrocarbons).
Additionally, species that react with the OH radical in the atmosphere (e.g., CO, MeOH, CH2O, and other hydrocarbons) can thus prolong the atmospheric lifetime of CH4, a potent greenhouse gas.
Aerosol particles in the atmosphere can impact the climate directly by reflecting or absorbing incoming sunlight, or indirectly by altering the properties of clouds.
The lifetime of particles in the lower layers of the atmosphere is on the order of 1–2 weeks, depending on the properties of the particles and their environment [7, 8]. Particles that absorb light, such as soot particles / black carbon (BC) and brown carbon (BrC), can contribute to warming both while in the atmosphere and after being deposited on ice or snow [9].
Reducing SOX emissions from ship engines
On average, anthropogenic aerosol particles in the atmosphere are considered to have a net cooling effect on the climate, but the climatic effect depends on the type of particle and the environment, and there is significant uncertainty associated with the magnitude [7, 8]. There is a broad consensus that ship engine emissions, in particular SOx, lead to the formation of aerosol particles with a global net cooling effect on the climate [7, 10, 11].
From a health perspective, reducing SOx emissions from ship engines is desirable; however, this will also reduce the anthropogenic cooling effect on the climate [12, 13]. The relatively short lifetime of particles in the lower portions of the atmosphere is important to note in this context.
A significant reduction in SOx emissions from ship engines will reduce climatic cooling on a short-term scale (weeks to months), producing a net warming effect [11, 14]. On the other hand, the immediate cessation of anthropogenic CO2 emissions would not have a significant effect on the climate until decades later due to the pronounced climate-relevant ‘residence time’ of CO2 in the atmosphere [7], considered to be on the order of one century1.
Health and environmental effects of ship engine emissions
Gases like NOx, CO, MeOH, and CH2O have various properties that make them harmful or toxic to humans. Other gases (e.g., CH4) can serve as precursors to harmful gases (e.g., ozone). The negative effects on human health of high particulate concentrations in the atmosphere are also well documented [15].
Emissions from the shipping industry are particularly concerning in terms of health in the context of densely populated coastal areas near high volumes of shipping traffic [12]. All emission constituents in a particulate form (e.g., BC) or which serve as precursors to particulates (e.g., SOx) can be expected to pose a risk to human health [15], as indicated in Table 1.
Emission constituents can also negatively impact various environments, for example through acidification (e.g., CO2, SOx), eutrophication (e.g., NOx), reactive components harmful to plants (e.g., ozone production), and so on.
Emissions from methanol combustion in ship engines: a possible win for the environment
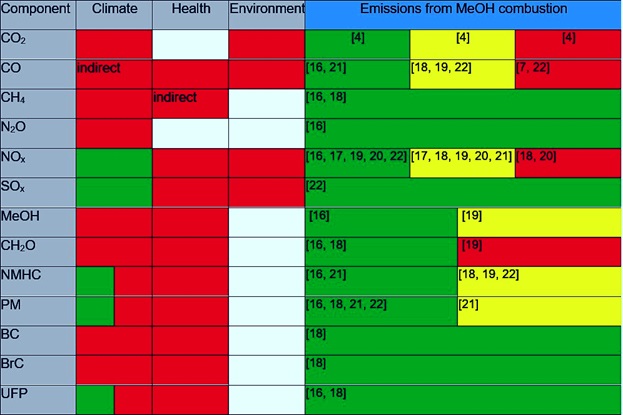
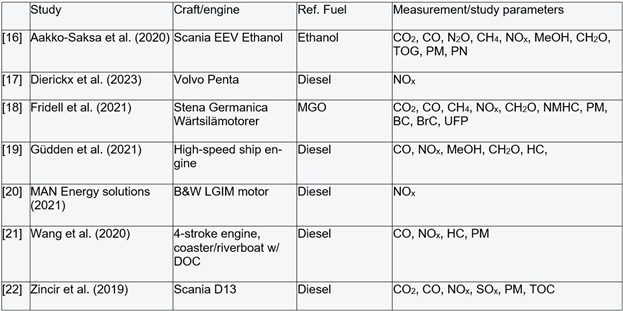
Only a limited number of scientific studies on emissions from methanol combustion in ship engines is available [16-22]. Furthermore, the proportion of relevant emission components whose concentrations are measured in these studies is typically low (see Table 2).
In the rightmost column of Table 1, we have indicated the expected emission levels for a variety of substances relative to their limit values (SOx and NOx) or relative to traditionally used fuels.
Across the board, we see significant reductions in both gaseous and particulate emissions when MeOH is used as fuel in ship engines. The major potential reduction in emissions may significantly benefit air quality and the environment in areas with a high volume of ship traffic.
The emissions in question may vary with the load on the engine (e.g., NOx), the pilot fuel and lubricants in use (e.g., SOx and particulates), and the combustion efficiency (e.g., MeOH and CH2O). The discussion that follows of potential challenges in using MeOH as fuel in ship engines will address (i) the climatic impact of emissions, (ii) NOx emissions, and (iii) formaldehyde (CH2O) emissions.
Table 1: An overview of potential components of combustion engine emissions in the shipping industry. The three central columns indicate whether a given component impacts the climate, human health, and/or the environment positively (green) and/or negatively (red). There is some overlap amongst several of the included emission components. NMHC is non-methane hydrocarbons. PM is particulate matter. BC is black carbon (soot particles). BrC is brown carbon: brown, carbonaceous particulate components. UFP is ultrafine particles (particles with a diameter < 100 nm). The rightmost column indicates the expected changes in emissions from transitioning to MeOH combustion in shipping. Green indicates a significant reduction in emissions and/or compliance with limit values for NOx and SOx, yellow indicates a more modest or ambiguous change, and red indicates a potential problem or unchanged emissions. An overview of the emission studies cited is presented below in Table 2.
Climatic impact of emissions from methanol combustion in ship engines
The assessment of the net climatic impact of the use of MeOH as a fuel in the shipping sector, we must account for potential positive and negative climatic impacts from (i) production, (ii) storage, (iii) transportation, (iv) spills, and (v) emissions from both MeOH and any pilot fuels and lubricants used.
There are other studies on the points i-iv, listed above [e.g. 4]. The following section focuses exclusively on (v) emissions, under the assumption that climate neutrality is achievable for points i-iv above.
As indicated in Table 1, a number of shipping emissions (SOx, NOx, and non-light-absorbing particles) have a net cooling effect on the climate. Beginning in 2020, the IMO introduced more stringent requirements for low sulphur content in ship fuels, from as high as 3.5% down to 0.5% outside of SECAs2. This change has reduced the climatic cooling effect of particles from the shipping industry [11, 14].
A more widespread transition to MeOH as fuel would be expected to further decrease the climatic cooling effect of particles produced by ship engines. Due to the relatively short lifetime of aerosol particles in the lower layers of the atmosphere, the reduced cooling effect would become immediately apparent.
Meanwhile, the reduced emission of greenhouse gases, including CH4, CO2, and N2O, would not significantly impact the climate for several years (CH4) to several decades (CO2, N2O) due to their longer atmospheric lifetimes.
Possible initiatives and timelines for relevant emission components
If we assume that ship engines could transition to burning climate-neutral (in terms of green-house gas emissions) MeOH in a short span of time, this will likely contribute to climatic warming for a few years, due to the reduced cooling from aerosol particle emissions. Meanwhile, the desirable climatic effects associated with reduced greenhouse gas emissions could be expected to become significant after several decades.
There may be several viable approaches to countering the effect of the expected warming associated with a major reduction in the shipping industry's emission of particulates. This may involve reducing emissions of warming short-lived climate forcers (SLCFs)3, such as BC and me-thane.
Thus far, the prevailing impression seems to have been that particulates emitted by the shipping industry have a net cooling effect [11, 12]. However, reducing BC emissions in particular is still a valuable focal point. The shipping industry's methane emissions have been growing through 2018, when these emissions amounted to 148 kt [3]. For comparison, the total anthropogenic emissions were in the vicinity of 360 Mt per year [23].
Thus, the contribution from the shipping industry apparently represented less than 0.5% of all anthropogenic emissions. In view of this, there are only limited prospects for reducing CH4 emissions. Nevertheless, doing so is still reasonable, and transitioning to MeOH as fuel could help in this respect.
Another potential focal point is reducing the emission of other short-lived climate forcers with indirect warming effects, such as CO and hydrocarbons, which increase the atmospheric lifetime of CH4 by reacting with OH. It is not clear whether MeOH becoming the industry's primary fuel would significantly reduce CO emissions, but there should be a potential for reducing hydrocarbon emissions nevertheless (see Table 1).
Prospects for carbon capture alongside the use of methanol as a green fuel for ship engines
In the relative short term, climatic cooling could be achieved if the introduction of MeOH made it possible to achieve significant negative CO2 emissions. Possible ways this could be achieved include the use of biomass in producing MeOH [4] and pilot fuels / lubricants combined with carbon capture on selected ships.
In short, a large-scale introduction of MeOH as fuel for ship engines could result in climatic warming over a period of a few years. Quantifying this effect precisely is challenging, given that there is significant uncertainty around the climatic effects of aerosol particles.
However, reductions in other emission constituents, or parallel capture and storage of atmospheric CO2, could at least partially offset likely warming effects over a few years to a few decades.
In the longer term (e.g., several decades), large-scale use of MeOH in ship engines would likely have a net cooling effect on the climate, provided that the entire process is optimised in terms of its climate footprint.
NOx emissions and the use of methanol as a new, green fuel
Across the board, most studies have found that the use of MeOH as a new, green fuel for ship engines would result in reduced NOx emissions compared to fossil fuels. In some contexts, NOx emissions may be low enough to satisfy the IMO's limit value for emission control areas (Tier III), although this may depend on the load and operation of a given engine [16, 17, 19, 20, 22].
A number of technologies exist to significantly reduce NOx emissions. For example, (i) methanol may be mixed with water [17, 20], (ii) a catalyst such as SCR (selective catalytic reduction) may be used, or (iii) EGR (exhaust gas reduction) or an equivalent technology may be used. Thus, it should be possible to reduce NOx emissions to favourable levels in terms of effects on human health and the environment.
Formaldehyde emissions and the use of methanol as fuel
Both very low [16, 18] and elevated emissions of formaldehyde (CH2O) have been reported from the combustion of MeOH in ship engines [19]. These emissions are most likely a result of in-complete combustion, due to either sub-optimal engine design or sub-optimal engine operation. Consequently, potential CH2O emissions for different engine loads should be studied.
Technological solutions (catalysts) exist that can minimise formaldehyde emissions, so these emissions should not necessarily disqualify MeOH as a fuel, nor disqualify a given engine from being adapted to burn MeOH.
However, efforts should be made to achieve complete combustion in engines; over time, optimised engine technology alone may be able to address any issues with formaldehyde emissions.
The expected positive impact of methanol in the long term
Large-scale use of MeOH as a new, green fuel for ship engines would be expected to significantly decrease the emission of various gaseous and particulate components. This would significantly benefit human health and the environment in turn.
However, such an initiative comes with the potential risk of increasing global warming on a timeline of up to a few decades, even if climate neutrality can be achieved in terms of MeOH production, storage, transportation, spills, and combustion.
This is due to the fact that through the present day, the shipping industry has emitted short-lived aerosol particles in significant quantities, and these particles have a cooling effect on the cli-mate. In the long term, the introduction of methanol as fuel in ship engines could help to reduce the atmospheric concentration of CO2, thereby contributing to a cooling effect on the climate.
1CO2 is regularly exchanged between the atmosphere and the biosphere/oceans, complicating the assessment of a meaningful climate-relevant estimate of the atmospheric lifetime of CO2. It has been assessed that if CO2 emissions were immediately reduced to zero globally, it would take nearly an entire millennium for the CO2 concentration in the atmosphere to naturally return to pre-industrial levels [24].
2Sulphur Emission Control Areas (SECAs) are subject to stricter requirements for low sulphur content in ship fuels. These are typically located near populated coastal areas, and they include all Danish waters.
3Constituents with an atmospheric lifetime under 20 years are typically considered "short-lived climate forcers" (SLCFs) [7]
References
[1] IMO, "https://www.imo.org/en/MediaCentre/HotTopics/Pages/Cutting-GHG-emissions.aspx," 2023. [Online].
[2] Liu et al., "A Perspective on the Ovararching Role of Hydrogen, Ammonia, and Methanol Carbon-Neutral Fuels towards Net Zero Emission in the Next Three Decades," Energies, vol. 16, 2023.
[3] Faber et al., "Fourth IMO GHG Study 2020," IMO, 2021.
[4] Kajaste et al., "Methanol-Managing greenhouse gas emissions in the production chain by optimizing the resource base," AIMS Energy, vol. 6, 2018.
[5] Bates et al., "The Global Budget of Atmospheric Methanol: New Constraints on Secondary, Oceanic, and Terrestrial Sources," J. Geophys.. Res.: Atmos, vol. 126, 2021.
[6] Millet et al., "New constraints on terrestrial and oceanic sources of atmospheric methanol," Atmos. Chem. Phys., vol. 8, 2008.
[7] Szopa et al., "Short-Lived Climate Forcers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change," 2021.
[8] Li et al., "Scattering and absorbing aerosols in the climate system," Nature Reviews Earth and Environment, vol. 3, 2022.
[9] Meredith et al., "Polar regions. chapter 3, ipcc special report on the ocean and cryosphere in a changing climate," 2019.
[10] Diamond et al., "Substantial Cloud Brightening from Shipping in Subtropical Low Clouds," AGU Advances, vol. 1, 2020.
[11] Watson-Paris et al., "Shipping regulations lead to large reduction in cloud perturbations," PNAS, vol. 119, 2022.
[12] Sofiev et al., "Cleaner fuels for ships provide public health benefits with climate tradeoffs," Nature Communcations, vol. 9, 2018.
[13] Bilsback et al., "Beyond SOx reductions from shipping: assessing the impact of NOx and carbonaceous-particle controls on human health and climate," Environ. Res. Lett., vol. 15, 2020.
[14] Yuan et al., "Global reduction in ship-tracks from sulfur regulations for shipping fuel," Science Advances, vol. 8, 2022.
[15] WHO, "WHO Air Quality Guidelines," 2021.
[16] Aakko-Saksa et al, "Renewable Methanol with Ignition Improver Additive for Diesel," Energy & Fuels, vol. 34, 2020.
[17] Dierickx et al., "Performance and emissions of a high-speed marine dual-fuel engine operating with methanol-water blends as a fuel," Fuel, vol. 333, 2023.
[18] Fridell et al., "Measurements of Emissions to Air from a Marine Engine," Journal of Marine Science and Application, vol. 20, 2021.
[19] Güdden et al., "An experimental study on methanol as a fuel in large bore high speed," Fuel, vol. 303, 2021.
[20] MAN Energy Solutions, "The Methanol fuelled MAN B&W LGIM Engine," MAN Energy Solutions, 2021.
[21] Wang et al., "Investigation to meet China II emission legislation for marine diesel engine with diesel methanol compound combustion technology," Jurnal of Environmental Science, vol. 96, 2020.
[22] Zincir et al., "Investigation of Environmental, Operational and Economic Performance of Methanol," Journal of Cleaner Production, vol. 235, 2019.
[23] Saunois et al., "The Global Methane Budget 2000-2017," Earth Syst. Sci. Data, vol. 12, 2020.
[24] Seinfeld & Pandis, Atmospheric Chemistry and Physics. From Air Pollution to Climate Change, 2006.






